Abstract
TP53 is mutated in ~4% of IDH mutated newly diagnosed (ND) acute myeloid leukemia (AML) and the clinical and prognostic impact of IDH/TP53 co-mutated AML is not well defined.
We analyzed patients (pts) with IDH1, IDH2, and/or TP53 mutated, ND AML treated at our institution from 2012 to 2022. Multihit TP53 status was defined by 1) multiple TP53 muts 2) TP53 mut(s) and concomitant 17p/TP53 deletion 3) TP53 mut variant allele frequency (VAF)>50% suggesting mutation+ copy neutral loss of heterozygosity. Treatment (trt) regimens were classified as IDH-targeted therapy (IDHi), intensive chemotherapy (IC), or low intensity therapy (LIC). All regimens were employed with or without the addition of venetoclax (Ven) per treatment protocol.
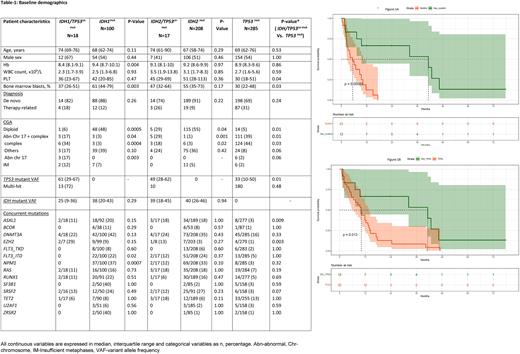
Among 628 pts with ND AML, 35 (5%) had IDH/TP53co-mut[23/35 (66%) IDH/TP53co-mut with multihit TP53], 100 (16%) had IDH1 mut, 208 (33%) had IDH2 mut, and 285 (45%) had TP53 mut [among 217/285 pts with evaluable TP53 allelic state, 185 (85%) had multihit TP53] (Table 1). Compared to pts withTP53 mut , pts with IDH/TP53co-mut had higher platelet count and higher bone marrow blasts and diploid karyotype (all p<0.05) more closely resembling features of IDHmut AML. However, pts with IDH/TP53co-mut had higher TP53 mut VAF [55%, interquartile range (IQR), (28%-67%)] compared with TP53 mut AML (33%, IQR, (10%-50%) (p=0.01).
The CR/CRi rates in IDH/TP53co-mut, IDH1 mut, IDH2 mut, and TP53 mut AML were 37%,77%,72%, and 48% respectively, regardless of trt regimen. Among pts with IDH/TP53co-mut, CR/CRi rates were significantly higher in non-multihit TP53 mut than pts with multihit TP53 mut (66% vs 9%, P=0.0005). Among pts who received Ven or IDHi based therapy, pts with IDH/TP53co-mut had significantly lower CR/CRi rates compared with pts with IDH mut AML (20% vs 87% ,respectively, P<0.05). In contrast, among pts treated with IC, pts with IDH/TP53co-mut had numerically superior CR/CRi rates compared with pts with TP53 mut (75% vs 47% vs respectively, P>0.05). Among pts treated with LIC, CR/CRi rates were similar between IDH/TP53co-mut , IDH mut ,and TP53 mut AML(31% ,48%,43%,respectively). The 60-day mortality rates in IDH/TP53co-mut, IDH1 mut, IDH2 mut, and TP53 mut were 11%,10%,7%,and 19%,respectively. Hematopoietic stem cell transplant rates in IDH/TP53co-mut, IDH1 mut, IDH2 mut, and TP53 mut AML were 17%, 20%, 25%, and 12%, respectively.
The median OS for pts with IDH/TP53co-mut, IDH1 mut, IDH2 mut, and TP53 mut was 8.7m,15.5m,30.3m, and 6.3m, respectively. The median OS was similar between those with IDH1/TP53co-mut and IDH2/TP53co-mut (7.8m vs. 9.5m; p=0.50). Compared to pts with IDH1 mut or IDH2 mut , those with IDH1/TP53co-mut (median OS-15.5m vs 7.8m) and IDH2/TP53co-mut (median OS-30.3 vs 9.5m) had inferior OS, respectively. There was no difference in OS between patients with IDH1/TP53co-mut and TP53 mut (median OS:7.8m vs 6.3m,p=0.3) but OS was superior in those with IDH2/TP53co-mut compared to those with TP53 mut (median OS 9.5m vs. 6.3m; p=0.03).
We additionally analyzed the outcomes of IDH/TP53co-mut and TP53 mut by multi-hit TP53status. Multihit TP53status was associated with inferior survival in those with IDH/TP53co-mut (median OS:5.4m vs 41.2m, p=0.0008) (Figure-1A) and TP53 mut (median OS: 5.8 m vs 10.1m, p=0.05) compared with non-multihit status. Among pts with non-multihit TP53 status, those with IDH/TP53co-mut had superior OS compared with those with TP53 mut AML (Median OS:41.2m vs 10.1m, P=0.01)(Figure-1B) Among pts who received IDH-sensitive therapy (i.e., Ven and/or IDHi), IDH/TP53co-mut had inferior survival compared with either IDH1 mut (median OS: 5.4m vs 24.4m, p<0.05) or IDH2 mut (median OS: 5.4m vs 35.5m, p<0.05) alone. Among pts who received Ven-based therapy, OS was similar in pts with IDH/TP53co-mut and TP53 mut (median OS: 5.4m vs 5.3m, p=0.72). Among pts who received LIC, there was no difference in OS between pts with IDH/TP53co-mut and TP53 mut (median OS: 5.0m vs 6.3m, p=0.92). However, among pts who received IC, IDH/TP53co-mut had superior OS compared with TP53 mut AML (median OS: 43.8m vs 5.1m, P=0.01). Notably all pts with IDH/TP53co-mut had non-multihit TP53 status.
IDH/TP53co-mut AML is a unique subtype with clinical features resembling IDHmut AML and prognosis driven by TP53 mut and the allelic status of TP53. Genomically sensitive therapies targeting both IDH and TP53 may improve the outcomes in this unique molecular subset of AML.
Disclosures
Loghavi:PeerView: Honoraria; QualWorld: Consultancy; Astellas: Research Funding; Abbvie: Consultancy, Current equity holder in publicly-traded company; GLG: Consultancy; Amgen: Research Funding. Kanagal-Shamanna:Amgen: Consultancy; Novartis: Consultancy; Aptitude Health: Speakers Bureau; Physicians Education Resource: Speakers Bureau. Issa:Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding; Novartis, Kura Oncology, Nuprobe: Consultancy. Sasaki:Otsuka Pharmaceuticals: Honoraria; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Takahashi:Ostuka Pharmaceuticals: Honoraria; Agios: Consultancy; Illumina: Honoraria; Mission Bio: Honoraria; GSK: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; Symbio Pharmaceuticals: Consultancy. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Ravandi:Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Prelude: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Syos: Consultancy, Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Amgen: Honoraria, Research Funding. Kantarjian:Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Daiichi-Sankyo: Consultancy, Research Funding; Pfizer: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Honoraria. DiNardo:AbbVie: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Bluebird Bio: Honoraria; Astellas: Honoraria; Foghorn: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; LOXO: Research Funding; Novartis: Honoraria; Takeda: Honoraria; Astex: Research Funding; Cleave: Research Funding; Forma: Research Funding; Gilead: Honoraria; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal